By Lisa Kaschmitter, Arizona State University Nutrition Student
Have you heard about the newest sweetener to arrive in our U.S. market? About a year ago, the Food and Drug Administration (FDA) approved the introduction of advantame. Advantame is a new artificial ingredient boasting 20,000 times the sweetness of regular table sugar (gram per gram). 1 High-intensity sweeteners, (as they have been dubbed by the industry), are nothing new. Most Arizonans are familiar with seeing other FDA approved artificial sweeteners such as Equal, Splenda, and Sweet’N Low available on the table at restaurants, as well as a familiar staple in many homes.
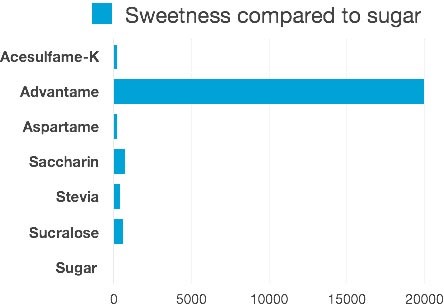
Graph source: blog.fooducate.com
Consumers and companies may consider the approval of this sweetener a win-win because this sweetener is a low-calorie sweetener and because of its potency, less will be needed to create a product, therefore lowering company production costs.2 Another consumer benefit that the FDA notes is that these high-intensity sweeteners typically do not raise blood sugar levels which helps companies cater products to people who suffer from illnesses that are effected by sugar intake and insulin levels, such as diabetes.2
What is Advantame?
Advantame is water-soluable and free flowing which means it is available to be used as a tabletop sweetener, while also having applications in baking, beverages, frozen desserts, and many more areas where sweetener is used.2 The advantame website states that:
“The U.S. Food and Drug Administration has approved Advantame, an innovative new sweetener, for general use in foods and beverages. Due to its excellent taste and functionality along with very low cost in use, advantame can be used to partially replace sugar, high fructose corn syrup or other high potency sweeteners. Since advantame blends well with caloric and non-caloric sweeteners, it provides food and beverage companies with an opportunity to reduce calories and manage their sweetness costs. Advantame extends sweetness duration in chewing gum and improves the sweetness profile of many confections. Maintaining the same sweet taste profile with less caloric sweetener can now be achieved with Advantame”3
The FDA cites the data from 37 animal and human studies to show advantame is a safe alternative to traditional sugar.1 These studies researched effects on the reproductive system, immune system, developmental system, and nervous systems.1 Although these tests showed no negative effects, many people still have concerns over the long-term effects. There were 4 human trials analyzed by the FDA, the longest of which, running for a 12-week time frame.4 These studies also were noted for their small sample size.4 Scientists agree that a larger sample size lead to more sound application of results.
Advantame contains phenylalanine, which is also a component of aspertame. Aspertame has been linked to many negative health effects if an individual consumes it over many years. Phenylalanine also can be difficult to metabolize for people with Phenylketonuria, (a rare genetic disorder). 5 The FDA attempts to clear concerns about both issues by reminding consumers that advantame is so sweet that it can be used in smaller amounts to produce the same sweet effect that aspertame needs larger quantities for and that artificial sweetener is just one of many sources of phenylalanine in the diet.4
One concern of those who suffer from Phenylketonuria is that advantame does not have to be labeled as an ingredient in products that use it.6 Their reasoning for this is that the levels of advantame that will be used to create an individual product are so small, that labeling is not necessary for public safety.6 This lack of labeling will make it hard for people who wish to track their intake, to know how much of an ingredient is being consumed during their daily lives.
Currently advantame is approved for use in the the United States, Mexico, the E.U., Austrailia, New Zealand, South Korea, and Taiwan.3
References
- Additional Information about High-Intensity Sweeteners Permitted for use in Food in the United States. U.S. Food and Drug Administration. FDA, 25 May 2016. Web. 23 November 2015.
- FDA Approves New High-Intensity Sweetener Advantame. U.S. Food and Drug Administration. FDA, 19 May 2014. Web. 23 November 2015.
- Advantame. Ajinomoto. Ajinomoto. n.d. Web. 24 November 2015.
- Food Additives Permitted for Direct Addition to Food for Human Consumption; Advantame. Federal Register. FDA. 21 May 2014. Web. 24 November 2015.
- FDA Approves New Artificial Sweetener. Melissa Healy. Los Angeles Times. 21 May 2014. Web. 24 November 2015.
- Coming your way- aspartame on steroids. Jenny Thompson. Dr. Leonard Coldwell. Web. 24 November 2015.

